Corneal Transparency in Dogs & Cats: Layers, Hydration & Nutrition
Corneal Transparency in Dogs & Cats
The cornea serves not only as the principal refractive element of the canine and feline eye, contributing approximately two-thirds of total dioptric power, but also as a biological barrier uniquely exposed to environmental, immunological, and iatrogenic challenges.
Its transparency is neither accidental nor passive. Rather, it is the result of a delicate equilibrium between the optical properties of collagen, the active regulation of tissue hydration, and the constant requirement for metabolic support in the absence of intrinsic vasculature. Even minor disruptions in this equilibrium—whether due to trauma, infection, metabolic derangement, or age-related degeneration—can lead to vision-threatening opacity. Therefore, understanding the determinants of corneal clarity is not merely an academic exercise but a clinical necessity.
Corneal Structure: Macro- and Microanatomy
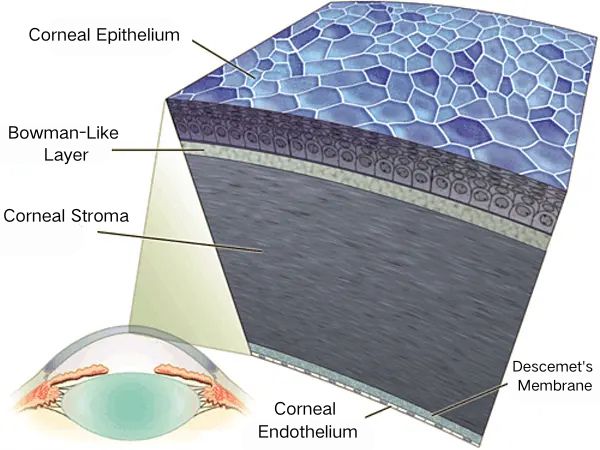
Epithelium and Bowman-Like Layer
At the surface, the corneal epithelium provides a dynamic interface between the external environment and the deeper ocular tissues.
In both dogs and cats, this non-keratinized, stratified squamous epithelium is five to seven cell layers thick, anchored to the underlying Bowman-like layer by robust hemidesmosomal attachments.
Tight junctions among superficial epithelial cells create a formidable barrier to pathogen entry and paracellular fluid ingress, a feature essential for preventing stromal swelling and maintaining a smooth optical surface.
Damage to the epithelial barrier, even at the microstructural level, can rapidly precipitate fluid imbalance and compromise transparency.
Notably, the regenerative capacity of the canine and feline corneal epithelium enables rapid repair following superficial injury, although chronic defects are a hallmark of underlying tear-film instability or systemic disease.
Stroma
The stroma constitutes approximately ninety percent of the corneal thickness and is responsible for most of its refractive and mechanical properties.
It is composed of over 200 lamellae of type I collagen, which are arranged in an orthogonal lattice—a design that is fundamental to minimizing light scatter.
In dogs, the average stromal thickness is roughly 0.55 mm, while in cats it is slightly less, about 0.50 mm; both species exhibit a remarkably uniform collagen fibril diameter of about 30 nm, and the precise, regular spacing of these fibrils (about 60 nm center-to-center) is governed by a matrix of keratan sulfate and chondroitin sulfate proteoglycans .
It is this nanoscale regularity that allows for constructive light transmission and explains why even minute changes in hydration, as seen with endothelial dysfunction or inflammatory disease, can result in visible haze or opacity.
Keratocytes, the resident stromal fibroblasts, play a central role in maintaining extracellular matrix composition and transparency; their response to injury or infection—marked by proliferation and increased production of matrix metalloproteinases—can be both reparative and, if unregulated, deleterious.
Descemet’s Membrane and Endothelium
Descemet’s membrane is an acellular basement membrane secreted by the underlying corneal endothelium.
With age, this membrane thickens, particularly in dogs, and while it provides structural support, it is the endothelium itself—a single layer of hexagonal, mitotically quiescent cells—that is chiefly responsible for maintaining corneal deturgescence.
These cells use a coordinated system of Na⁺/K⁺-ATPase and Cl⁻/HCO₃⁻ exchangers to actively transport ions (and thus water) from the stroma to the aqueous humor.
Endothelial cell density, averaging 2600–3000 cells/mm² in young dogs and cats, declines steadily with age or disease.
When density drops below a critical threshold (~500 cells/mm²), the endothelial pump’s reserve is lost, and irreversible stromal edema (bullous keratopathy) develops—a condition particularly prevalent in older dogs and less common, but not unknown, in geriatric cats .
Corneal Hydration and Homeostasis
Fluid Dynamics and Pump-Leak Model
Corneal transparency is intimately tied to the precise regulation of tissue hydration.
While the proteoglycan-rich stroma has a natural tendency to imbibe water, the continuous activity of the endothelium’s metabolic pumps ensures that excess fluid is constantly moved posteriorly into the anterior chamber.
The so-called“pump-leak”hypothesis captures this balance: passive fluid influx (leak) is counteracted by active ion-coupled extrusion (pump), with aquaporins facilitating rapid water movement across cellular membranes.
Species differences exist in endothelial robustness; for example, some canine breeds, such as Boston Terriers and Chihuahuas, are genetically predisposed to premature endothelial cell loss, whereas similar disorders in cats are rare and often secondary to chronic uveitis.
Role of the Tear Film
The tear film contributes to corneal clarity by providing nutrients, oxygen, and antimicrobial peptides, while also washing away debris and maintaining epithelial moisture.
It is a trilaminar structure composed of a superficial lipid layer (from Meibomian glands), an aqueous layer (from lacrimal and nictitating membrane glands), and a mucin layer (from conjunctival goblet cells).
Disruption of any tear-film component—be it from KCS, eyelid malposition, or conjunctival disease—impairs the epithelial barrier and predisposes to stromal dehydration or overhydration.
In closed-eye conditions, the tear film supplies up to 20% of the corneal oxygen requirement, while in the awake state, atmospheric oxygen diffuses directly through the tear film and epithelium.
Metabolic and Nutritional Considerations
Although avascular, the cornea is highly metabolic, relying on glucose from the aqueous humor and oxygen from the tear film and atmosphere.
Keratocytes predominantly employ anaerobic glycolysis, but the endothelium is heavily dependent on mitochondrial oxidative phosphorylation to fuel the Na⁺/K⁺-ATPase pumps.
Antioxidant defenses—superoxide dismutase, catalase, ascorbate—are crucial for neutralizing reactive oxygen species generated by ultraviolet light and metabolic activity.
Importantly, lactate generated by aerobic glycolysis is co-transported with protons out of the stroma; if lactate accumulates, osmotic water retention ensues, leading to haze.
Pathophysiological Perturbations
Disturbances in any layer or metabolic process threaten corneal transparency.
Corneal edema, most commonly due to endothelial decompensation or persistent epithelial defects, increases stromal thickness and disrupts collagen organization, manifesting as the familiar blue-white “ground-glass” opacity.
Hereditary dystrophies—seen in certain canine breeds—cause abnormal deposition of lipids or mucopolysaccharides within the stroma, presenting as crystalline opacities that may or may not affect vision.
Ulcerative keratitis, in which enzymatic degradation of the stroma by neutrophil-derived proteases predominates, rapidly leads to loss of lamellar order and catastrophic light scatter.
Finally, chronic or severe inflammation may irreversibly deplete keratocyte populations or result in fibrotic scar formation.
Diagnostic Approach
Clinical evaluation of corneal transparency must be systematic.
Slit-lamp biomicroscopy allows for detailed inspection of epithelial integrity, stromal haze, and Descemet’s folds, while retroillumination techniques can highlight subtle opacities.
Quantitative assessment with ultrasonic pachymetry establishes baseline and progressive changes in stromal thickness; confocal microscopy, though less widely available, offers in vivo assessment of keratocyte and endothelial health.
Fluorescein staining remains invaluable for detecting epithelial compromise, whereas fluorophotometry and specular microscopy—though primarily research tools—may in future become more widely used in clinical practice.
References
Maggs DJ, Miller PE, Ofri R. Slatter’s Fundamentals of Veterinary Ophthalmology. 4th ed. Elsevier; 2008.
Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. 6th ed. Wiley-Blackwell; 2021.
