Fluorescein Sodium and Rose Bengal Staining: Usage and Precautions
Ophthalmic dye test strips, as one of the fundamental diagnostic tools in veterinary ophthalmology, hold significant value in identifying various common ocular surface diseases such as corneal diseases, tear film abnormalities, and nasolacrimal duct obstruction. This article systematically reviews the physicochemical properties, clinical indications, and operational considerations of fluorescein sodium and Bengal rose.
Fluorescein Sodium Test Strips
Fluorescein sodium is the most widely used dye in veterinary ophthalmology. Its significant water solubility and lipophobic nature make it an ideal tool for evaluating ocular surface integrity and tear film dynamics. Its absorption peak is at 490 nm, and it exhibits a green fluorescence (520 nm) in an alkaline environment, which is most clearly observed through a cobalt blue filter.
Fluorescein can be applied topically either as a 2.0% solution or via impregnated dye strips. Due to the potential for bacterial contamination, strict adherence to single-dose, sterile packaging should be maintained during clinical procedures.
Detection of Corneal Epithelial Defects and Ulcers
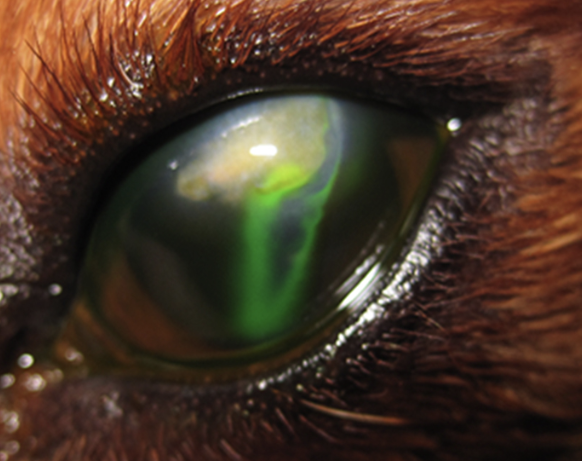
The most fundamental application of fluorescein sodium is to assess the integrity of the corneal epithelium. As it cannot penetrate the lipid barrier of healthy corneal epithelium, the dye only adheres to exposed corneal stroma, appearing as a bright green fluorescence under cobalt blue light. This characteristic makes it the preferred tool for diagnosing superficial corneal ulcers.
During use, direct contact of the dye strip with the corneal surface should be avoided to prevent mechanical damage leading to false positives. After staining, thoroughly rinse off residual dye with sterile saline to prevent misinterpretation caused by dye retention or “pooling” on irregular corneal surfaces.
Note: Fluorescein does not stain Descemet’s membrane. Thus, in deep corneal ulcers, if no staining is seen at the lesion’s base, a descemetocele should be suspected.
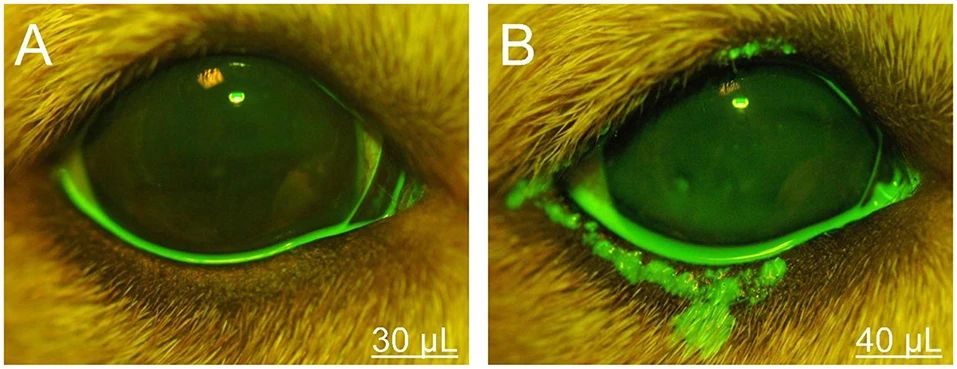
Seidel Test: Assessment of Corneal Perforation
The Seidel test is a classic method that utilizes fluorescein to detect aqueous humor leakage, used to assess for perforating corneal injuries.
This test should be performed with a high concentration of unrinsed dye, allowing for the identification of dynamic changes as the dye is diluted by aqueous humor. As aqueous humor leaks from a corneal defect, it forms a pale green “stream” that diffuses outwards from the center against the fluorescein background, particularly evident under a slit lamp or direct ophthalmoscope.
The Seidel test holds significant value in areas such as postoperative corneal wound monitoring and the assessment of spontaneous corneal rupture, often serving as the preferred examination method, especially in emergency trauma cases. Notably, excessive tear or mucus coverage on the ocular surface can also impair the accuracy of Seidel test observations.
Tear Break-Up Time (TBUT) Measurement
TBUT is the standard method for evaluating tear film stability, indirectly reflecting the integrity of the mucin and lipid layers within the tear film.
During the examination, after applying fluorescein to the corneal surface, allow the animal to blink naturally several times. Then, hold the eyelids open and record the time from the last blink until the first dry spot appears on the tear film surface. Perform three consecutive tests and calculate the average time.
Normal canine TBUT ranges from 19.7 to 21.5 seconds, while for felines it is 12 to 21 seconds. A significantly shortened TBUT typically indicates decreased tear film quality and can be observed in various conditions such as keratoconjunctivitis sicca (dry eye), viral keratoconjunctivitis, and corneal degeneration. It is important to note that fluorescein dye itself may cause some disturbance to the tear film; therefore, consistent dye concentration and procedural methods should be maintained during each test to obtain comparable data.
Jones Test: Assessment of Tear Drainage Function
The Jones test is used to evaluate the functional patency of the nasolacrimal duct system and is a fundamental method in screening for lacrimal drainage disorders. After fluorescein is instilled into the conjunctival sac, and without rinsing, it is observed whether the dye can flow through the lacrimal drainage system to the ipsilateral nostril or nasolabial groove skin within a specified time.

Fluorescein Sodium & Rose Bengal
Normal canine fluorescein passage time varies from 30 seconds to 5 minutes, with cats typically exhibiting faster passage. If no dye is observed to exit within 10 minutes, the possibility of nasolacrimal duct obstruction or anatomical variation should be considered.
In brachycephalic breeds, due to the flattened and short structure of the nasolacrimal duct, the dye often drains directly into the nasopharynx instead of the external nostril, which may lead to false-negative results. Therefore, Jones test results should be comprehensively evaluated in conjunction with clinical signs, nasolacrimal duct flushing, and diagnostic imaging.
Bengal Rose Test Strips
Bengal rose staining complements fluorescein’s function by primarily staining corneal cells that lack a protective mucin layer. This examination offers reference value for diagnosing corneal epithelial defects, abnormal mucin, and dry eye syndrome. After instilling a 1% solution into the animal’s conjunctival sac, allow it to blink 3-5 times, then observe for conjunctival lesions. As this reagent has some irritating properties, local anesthesia should be administered prior to the examination, and the ocular surface should be rinsed after the test to prevent residual reagent from causing irritation to the eye.
In clinical practice, it can be used either before or after fluorescein staining, as the two do not interfere with each other.
Fluorescein Sodium & Rose Bengal
Additionally, another commonly used dye is Lissamine Green, which is considered a true vital stain. It does not stain healthy cells and its staining pattern is similar to that of Bengal rose, making it a potential alternative dye, though it is less commonly used in veterinary clinical practice.
Precautions
Dye application can interfere with the laboratory diagnosis of infectious diseases; therefore, relevant microbiological sampling should be completed prior to any staining procedure. Fluorescein can promote the growth of certain bacteria and interfere with immunofluorescence assays, while Bengal rose has known antibacterial and antiviral activity, which can lead to false-negative results in culture or PCR tests. To ensure the accuracy of test results, sampling and staining procedures should be strictly performed in separate steps in clinical practice.
References
All data and quotations are from:
Gelatt KN, editor. Veterinary Ophthalmology. 6th ed. Hoboken (NJ): Wiley-Blackwell; 2021.
Vet Times. Corneal ulcers in dogs: causes, signs and treatment [Internet]. Peterborough: Veterinary Times; 2023 [cited 2025 Jun 12]. Available from https://www.vettimes.com/news/vets/small-animal-vets/corneal-ulcers-in-dogs/
Yokoi N, Georgiev GA, Kato H, Komuro A, Maruyama K, Shigeyasu C. Characteristics and utility of fluorescein breakup patterns among dry eyes in clinic-based settings. Int J Ophthalmol. 2020;13(12):1946–1953.
Williams DL, Burg P, Smith K. Tear film breakup time and Schirmer tear test in normal dogs: Effects of age, sex, reproductive status, skull type, and nasolacrimal duct patency. Vet Ophthalmol. 2004;7(2):91–96.

